
Researchers at Boston University are working to develop a drug, and a delivery system, that may help prevent blood vessels from bursting and causing early-stage dementia.
While Alzheimer’s disease is likely the most well-known form, dementia comes in other, lesser-known varieties, like “vascular dementia”—caused when tiny blood vessels burst in the brain, leading to micro-strokes and minute bleeds. The resulting condition is closely linked with other age-related memory disorders.
“I suspect that vascular dementia and Alzheimer’s are really just two different angles on the same disease,” says Kathleen Morgan, professor of health sciences at Boston University College of Health & Rehabilitation Sciences: Sargent College. “There are plaques in the brain tissue of Alzheimer’s patients that are visible at autopsy, but we know that if you have them, you’ll probably see evidence of microbleeds as well.”
And if burst blood vessels are implicated in early-stage dementia, Morgan says, it may be possible to stop that damage before it starts.
With a $2.5 million grant from the National Institute on Aging (NIA), Morgan is examining a synthetic prototype drug that could prevent microbleeds in mouse brains. To do so, she’s joining forces with Tyrone Porter, associate professor of mechanical engineering at BU, working across disciplines to develop a new delivery system for the drug.
Their solution uses a novel system of “microbubbles”—tiny bubbles of inert gas smaller than capillaries—along with a focused ultrasound beam to help push the drug into a very specific part of the body: the large blood vessels next to the heart.
Most brain bleeds, Morgan says, actually start in the aorta, the body’s largest artery, which connects directly to the heart. With each beat, the heart exerts enormous amounts of pressure straight onto that conduit, which is made of smooth muscle cells that expand and contract like a rubber hose as blood flows past them. “Those smooth muscle cells are very important for controlling the pressure of your vascular system on a beat-to-beat basis,” she says.
In younger bodies—both mouse and human—smooth muscle in the aorta expands with each beat, acting as a sort of “shock absorber” for the pressure coming out of the heart. In older bodies, though, it becomes gradually less elastic, meaning that the energy of each pulse travels further through the vascular system. If the aorta becomes stiff enough, blood can surge at high pressure straight into tiny, sensitive blood vessels in the brain, which may burst under the strain.

Morgan holds a vial containing a mouse brain. The brain, removed from an aged mouse, is comparable to the brain of a 75-year-old human. Morgan and her collaborator Porter are using mice to test a prototype drug that could prevent stroke-causing microbleeds. Photo by Jackie Ricciardi
Morgan is developing new ways to reverse the stiffening of arteries. If she can restore some of the aorta’s elasticity, she reasons, it may be possible to prevent new microbleeds. To test this idea, she’s concocted a new peptide—a small chain of amino acids—that can control smooth muscle stiffness.
In smooth muscle tissue, she says, elasticity is determined in part by two types of long, stringy molecules called actin and myosin, which form a web inside each cell. As the two strands latch onto each other, they restrict the cell’s movement, stiffening its structure. “It’s a bit like a Chinese finger trap,” says Morgan. “The harder you tug on actin, the harder it clamps down.” The peptide her team has created, however, can effectively stop this process in its tracks by binding to the molecules, preventing them from grabbing onto their counterparts in the first place. As a result, the cell remains relaxed and supple. Morgan can control how stiff or loose the tissue gets by controlling the amount of peptide she administers.
The challenge, she says, is delivering those molecules directly to the smooth muscle inside a living aorta. Unlike other drugs, releasing this one system-wide—or even artery-wide—could be disastrous. “Smooth muscle tissue isn’t just in the aorta. It’s in your vascular system, urinary tract, uterus, lung tissue, and digestive system,” she notes. “If the peptides got into those tissues, it could cause incontinence, premature labor, all sorts of awful things.”
To get the drug exactly where it’s needed, however, you first have to dig into the artery itself.
“The cells we need to target don’t come in contact with flowing blood. They’re behind a layer or two of other cells and connective tissue in the blood vessel walls,” says Porter. In order to break through those layers and deliver the drug directly to smooth muscle cells, he’s attaching Morgan’s peptides directly to the outside of each microbubble. Focused ultrasound can be used to push the microbubbles toward the aortic wall and pop them to release the peptide. The “popping” process also subtly and reversibly disrupts the lining of the aorta, making the blood vessel wall temporarily permeable. “Once that happens, the peptide can flow directly into the spaces that open up in the vessel wall, and go straight into the smooth muscle tissue,” Porter says.
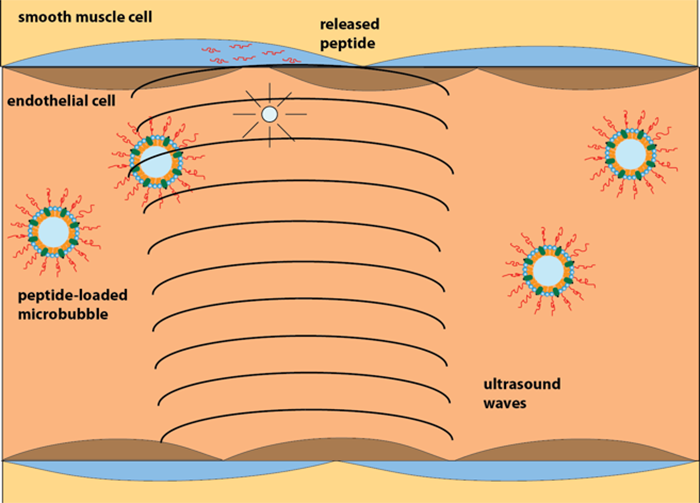
Morgan and Porter’s drug delivery system. The scientists attach the drug to microbubbles smaller than capillaries and use a focused ultrasound beam to guide the bubbles and then burst them, delivering the drug directly into smooth muscle cells. Eventually, they hope their system may be used to deliver drugs to the large blood vessels next to the heart. Illustration by Tyrone Porter.
These microbubbles themselves are simple to make, he adds, and the FDA has already approved them for use. “Microbubbles have been used for years as contrast agents for ultrasound. They scatter sound much better than tissue, so they’re used to distinguish blood from the chambers in heart and surrounding muscle,” he says. They’re also tiny enough to fit through the smallest blood vessels in the body and eventually disappear as the gas, which is harmless, escapes into the blood and is expelled out of the body through the lungs.
In addition to being relatively safe, the clinical advantage of this approach is that it can be done with a standard ultrasound probe commonly used in a cardiac echo test. Using a low-powered ultrasound beam, a technician can track where the bubbles are going, then pop them at a specific location by simply turning up the strength of the beam. “[Existing ultrasound tools] can focus the beam down to the millimeter, so it’s extremely accurate,” says Porter.
Until now, Morgan has only been able to test her peptide and its new delivery system on smooth muscle cells in a petri dish. With the new grant from the NIA, however, she and her collaborators are looking to scale up their research, and they will use their approach for the first time on a living animal.
“My earlier work was just on the fundamental mechanics of these peptides. Moving into a whole mouse is a big leap for someone used to sitting at a bench dealing with cells!” she says, laughing. “The people I’ve connected with here at BU make it feasible, though. That’s how you get basic discoveries translated into practical ones—you have lots of scientists working in parallel. You need teams instead of a single investigator.”
By David Levin
Hearts, Minds, and Microbubbles was originally published on the Boston University website.