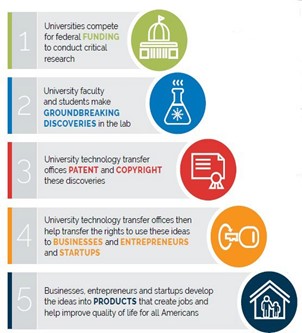
What’s at stake: By enabling universities to patent and license groundbreaking discoveries, the Bayh-Dole Act of 1980 significantly improved the U.S. system of technology transfer. Now proposals to reinterpret when Bayh-Dole “march-in rights” can be used are threatening the future of university technology transfer.
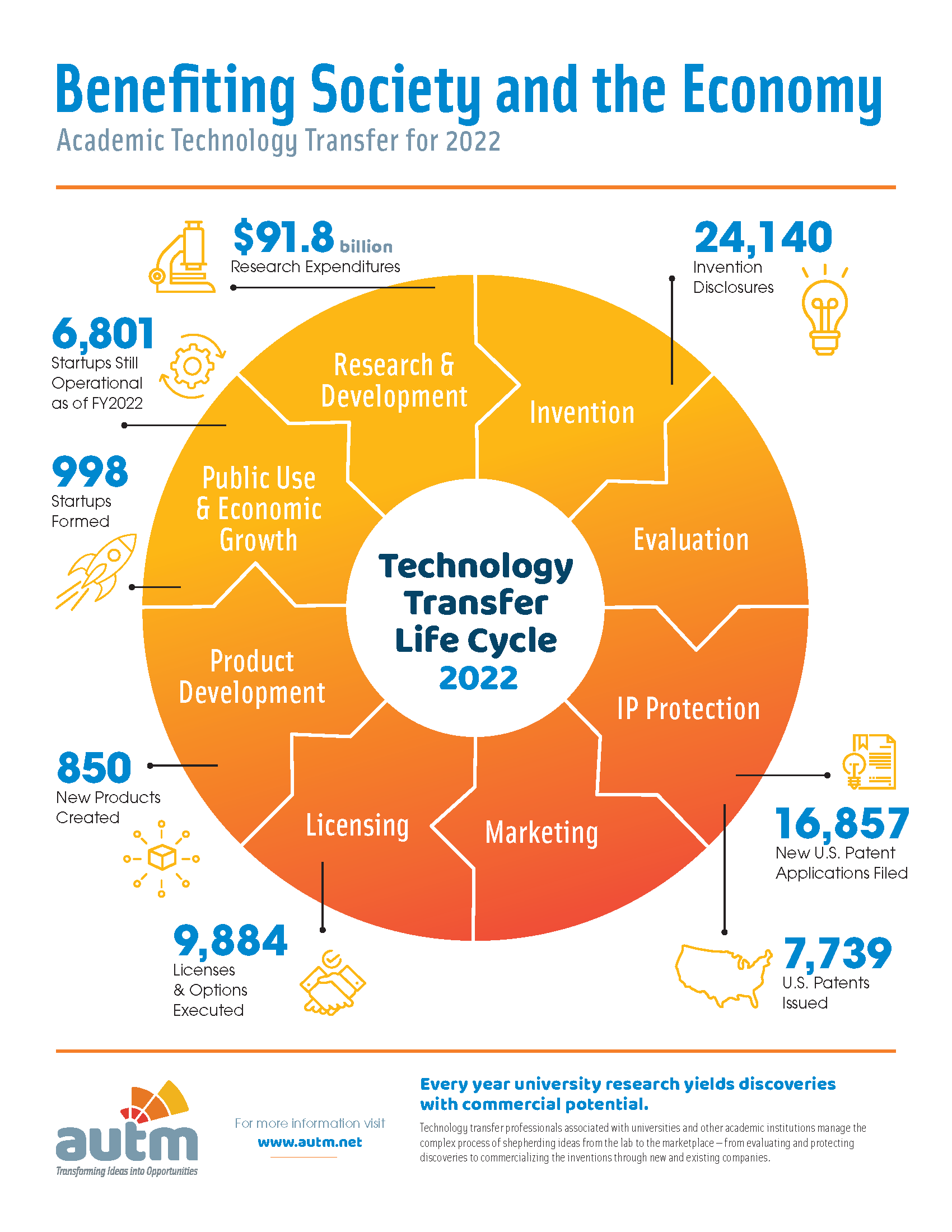
Key Facts:
- Congress built safeguards into Bayh-Dole that enable the government to protect the public against nonuse or unreasonable use of federally supported inventions. The law grants the federal agency that funds the research a limited right to “march in” and require the owner of a patent that was developed through federal funding to grant additional licenses to the invention to ensure that the invention is made available to the public. Congress approved the march-in provision to address concerns that dominant companies would pay to license competitive university inventions solely to prevent their development and entry into the marketplace.
- The legislative record and subsequent statements by Senators Bayh and Dole make clear that Congress intended this march-in provision to apply only in narrow circumstances, such as when a licensee fails to make a good-faith effort to bring the invention to market or if health or other emergencies arise and the licensee is unable to make enough of a product to meet public needs.
- The march-in provision was never intended to serve as a vehicle for controlling drug Rather, the statute refers to “practical application,” which is defined as providing availability to the public on terms that are “reasonable under the circumstances.” The statute deliberately does not address or define what may constitute a “reasonable price.”
- Over the years, the National Institutes of Health (NIH) has considered and rejected several petitions asking it to exercise its march-in authority to address drug pricing concerns. In each review to date, NIH has concluded that the “practical application” requirement was satisfied if the patented drug was on the market and available to the public.
- For universities and their patent licensees, the exercise of march-in rights for reasons other than exigent health or safety needs would create substantial market uncertainty that would chill university technology If the scope of applicability of march-in rights is broadened beyond Congress’ original intent, the private sector will be hesitant or unwilling to license federally funded inventions from universities, potentially impeding progress against some of our costliest and most challenging diseases to the detriment of public health and safety.
Intellectual property rights make the existence of, and access to, critical medicines and medical technologies more likely, not less. Undermining Bayh- Dole and the important patent protections this landmark legislation provides will impede the development of innovative new medicines and medical technologies resulting in fewer – not cheaper – new drugs.